Welcome to “Naijaclass Academy” For Waec Chemistry Practical 2024 Expo Answer

Date: Thursday, 16th May, 2024
Chemistry Practical 9:30 am. – 11:30 am. (1st Set)
——————————
*TEACHERS REPORT FORM*
(2)
(a)
A contains *1.58* g of KMno4 per dm³ of solution
(b) B contains *5.5* g of FeSO4 per dm³ of solution
(3)
(i) 18.10 cm³
(ii) 18.20 cm³
(iii) 18.10 cm³
Average: 18.13cm³
2024 WAEC CHEMISTRY PRACTICAL ANSWERS
ATTENTION
Candidates must use their school’s endpoint in place of the 18.13cm³ used.
Use your end point also in b(i) to calculate concentration of B
NUMBER 1
Updated b(i)
Final answer approximately 0.1
—————————————————
(2a)
When all of C is added to a test tube with about
10cm³ of distilled water and shaken, the mixture is filtered. The residue and the filtrate are obtained.
Observations:
The residue: This will contain the insoluble salt(s) present in C.
The filtrate: This will contain the soluble salt(s) present in C.
(2bi)
Heating about 2cm³ of the filtrate in a boiling tube strongly.
Observations:
If there is effervescence (bubbling), it indicates the presence of a carbonate salt in the filtrate.
The gas evolved is likely carbon dioxide (CO₂).
(2bii)
Adding dilute HCl to half of the residue in a test tube.
Observations:
If there is effervescence (bubbling), it indicates the presence of a carbonate salt in the residue.
The gas evolved is likely carbon dioxide (CO₂).
(2biii)
Adding aqueous NaOH in drops, then in excess, to about 2cm³
Observations:
If a white precipitate forms upon adding NaOH drops, and it dissolves upon excess addition of NaOH, it indicates the presence of a metal hydroxide in the solution.
(2biv)
Adding aqueous ammonia in drops, then in excess, to another 2cm³ of the clear solution from (ii).
Observations:
If a colored precipitate forms upon adding ammonia drops, and it dissolves upon excess ammonia addition, it indicates the presence of a metal hydroxide in the solution.
====================================
(3a)
(PICK ANY TWO)
(i) Ammonia (NH₃)
(i) Oxygen (O₂)
(ii) Carbondioxide (CO₂)
(iii) Nitrogen dioxide (NO₂)
(iv) Chlorine (Cl₂)
(v) Hydrogen sulfide (H₂S)
(3b)
(PICK ANY TWO)
(i)Pipette
(ii) volumetric flask
(iii)burette
(iv) Retort stand
(3c)
(PICK ANY ONE)
(i) Fume cupboard is used to protect the user from inhaling harmful chemicals or toxic gases
(ii) Fume cupboard is used to prevent chemical spills or accidents from spreading outside the workspace.
(iii) Fume cupboard is used to capture and remove air-borne hazardous substances generated during laboratory experiments
(iv) Fume cap used to contain and exhaust fumes in laboratory
(3d)
(PICK ANY THREE)
(i) Lab Coats.
(ii) Gloves.
(iii) Face shield.
(iv) Safety goggles
(v) Chemical-resistant aprons.







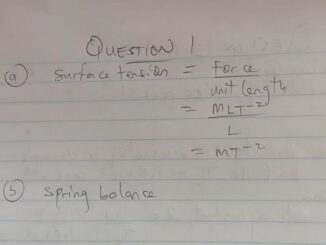

Am interested
I need it
Am interested
I’m interest
I am interested in chemistry practical
I want chemistry practical answers 2024
Tnx
Am interested
Am interested
AM interested
Am interest
I am Interested
I’m interested
I am interested
OK thnx
Am interested
Pls
I have paid for the chemistry practicals since yesterday evening but not yet given the pin.
Interested
Am in
Please just give me the expo but I will do everything leter okay
Thank u people for all this good work
I need chemistry obj
Am interested
as you dey help people.God will help you 2
Amen
as you dey help people God will help you 2
Literature question and answer please
I,m interested
NOT INTERESTED
Chemistry practical for set B